
Avogadro’s number can be used to understand how chemicals interact and how molecules within chemical samples interact. In calculations that involve more than one chemical, Avogadro’s number and moles must be used. It is done so that weight can be compared to the number of atoms within the substance (as defined by the number of moles) since weight is easier to measure than the number of atoms in the chemical sample. By representing 6.02214076 × 10 23 atoms, molecules are used in chemical calculations. The chemical reactions involve billions of atoms interacting with one another and being rearranged, but the movement of billions of atoms would be impossible to represent.Įven so, scientists still need a unit of measurement that can represent billions of elementary entities. In chemistry, Avogadro’s number and the mole are important concepts. Therefore, the number of atoms is 6.02214076 × 10 23, also known as Avogadro’s number. One mole of carbon 12 contains the same number of elementary entities as there are atoms. Mole counts are used by scientists to determine the number of elementary entities present in a chemical sample. A mole is a unit of measurement used to describe the quantity of a substance. In the case of moles, this definition isn’t very useful.

The molar mass of a chemical substance is the mass contained in one mole of that substance, the mass of one mole of a given substance. Add the masses of all the elements in the molecule to find the molecule’s mass. Multiply the atomic mass of an element by the number of atoms in the molecule to find the molecule’s mass. The molecular mass of an element is equal to the sum of the masses of its constituent elements. The formula mass of calcium hydrogen carbonate is 117.10 amu, and the molar mass of calcium hydrogen carbonate is 117.10 grams per mole (g/mol). We will use the term molar mass if we are discussing a mole of an ionic compound. Ionic compounds do not have individual molecules.
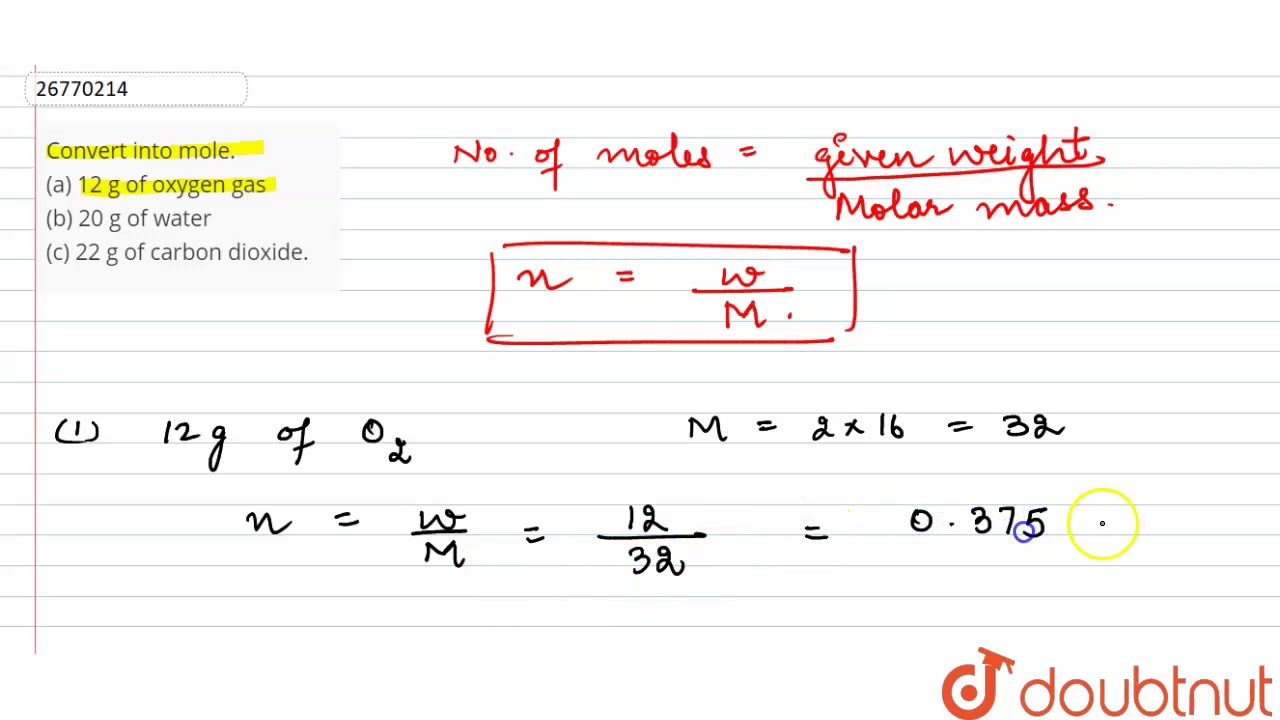
When referring to compounds that are not molecular (ionic compounds), the term “molecular mass” is improper, and “formula mass” is generally used instead. Consequently, N2 has a molar mass of 28.02 grams per mole. The molecular mass of a molecule is the result. (i) Your answer for part (h) should be three times as large as the answer for part (g).The atomic mass of an element is the sum of the atomic masses of all its constituent atoms. What is the average force the molecule exerts on one of the walls of the container? (Assume that the molecule’s velocity is perpendicular to the two sides that it strikes.) (f) What is the average force per unit area? (g) How many oxygen molecules traveling at this speed are necessary to produce an average pressure of 1 atm? (h) Compute the number of oxygen molecules that are contained in a vessel of this size at 300 K and atmospheric pressure. What is (a) the average translational kinetic energy of an oxygen molecule at a temperature of 300 K (b) the average value of the square of its speed (c) the root-mean-square speed (d) the momentum of an oxygen molecule traveling at this speed? (e) Suppose an oxygen molecule traveling at this speed bounces back and forth between opposite sides of a cubical vessel 0.10 m on a side.
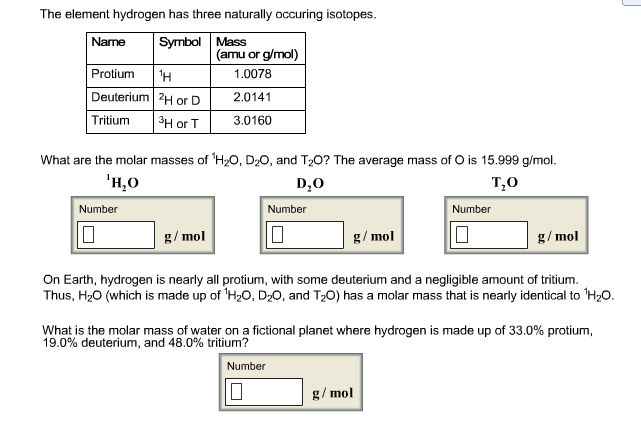
Oxygen ( O 2 O_ O 2 ) has a molar mass of 32.0 g/mol.


 0 kommentar(er)
0 kommentar(er)
